Rapamycin is a paradoxical drug. It improves anti-cancer immunity, but it may also cause cancer. It protects against bacterial infection and halts viral replication, but it also suppresses the immune system. It reverses symptoms of Alzheimer’s disease but also increases plaque deposits in the brain. It improves metabolic function, but it also causes type 2 diabetes. This list of contradictions goes on and on; however, there is one thing about rapamycin that scientists tend to agree on: it is potentially the most powerful anti-aging drug ever discovered. Unfortunately, rapamycin has been unlucky.
Rapamycin as an antifungal
In the 1960s, the government of Chile planned to build an international airport on one of its more popular territories, Easter Island. Two Canadian scientists, Stanley Skoryna and Georges Nogrady, viewed this as the perfect opportunity to study the island and its people before and after the airport’s construction. In addition to conducting extensive physical exams on most of the island’s population of approximately 1,000 individuals, Skoryna and Nogrady collected soil samples from different parts of the island to characterize the diversity of microorganisms. Ultimately, Skoryna and Nogrady’s enterprise yielded little academic reward. However, their soil samples somehow ended up in the hands of Surendra Sehgal, a senior researcher at Ayerst Pharmaceuticals, now Pfizer.
In 1972, the Ayerst team, led by Sehgal, identified a new antifungal compound in the soil samples.The compound was named rapamycin, in homage to the Polynesian name for Easter Island, Rapa Nui.
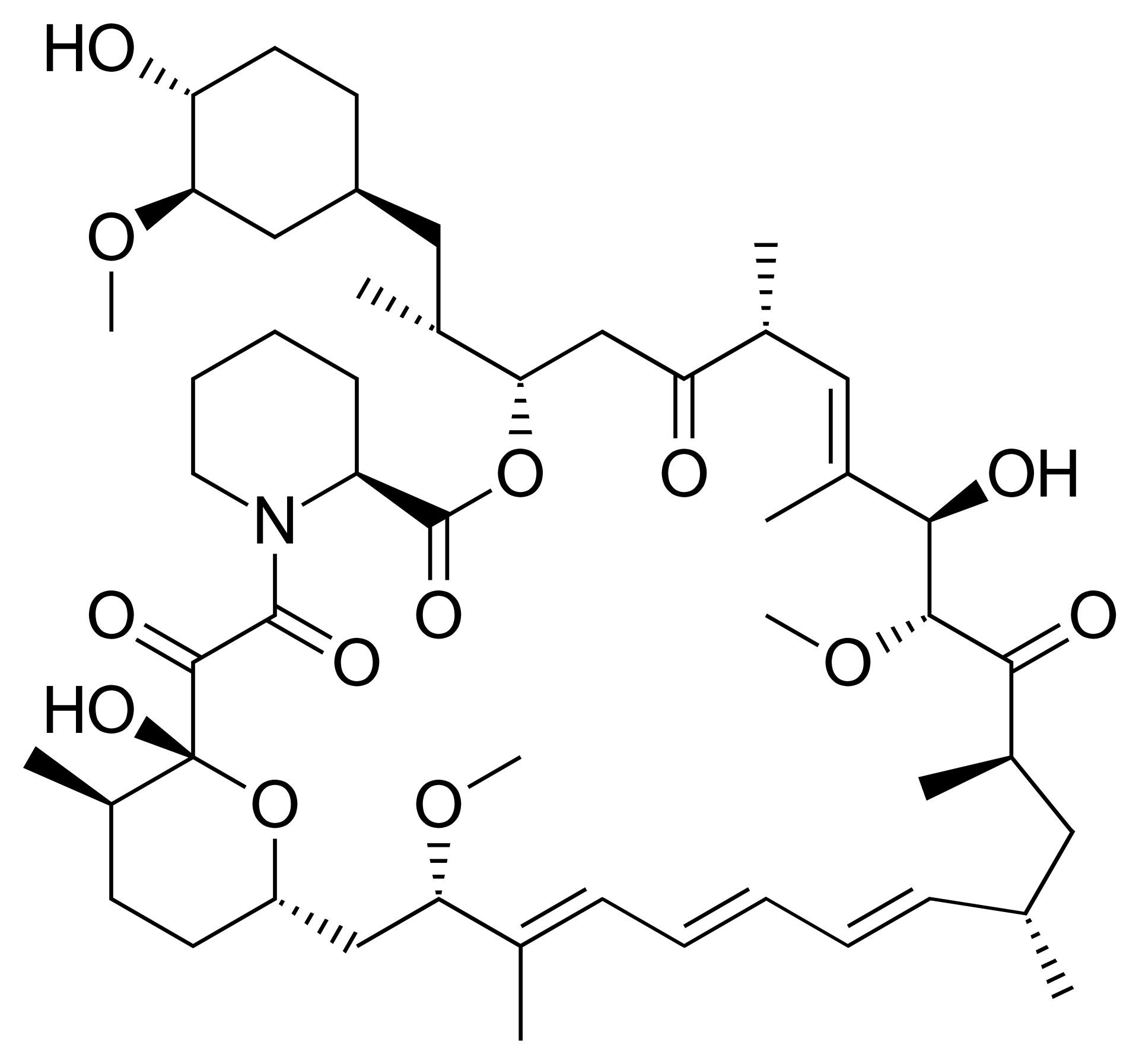
Unfortunately, rapamycin’s antifungal potential was short-lived. Sehgal and his team quickly learned that rapamycin blocked the production of immune cells, an undesirable characteristic for a patient trying to fight off an infection. The drug was labeled an immunosuppressant, and that put an end to its antifungal adventure.
Rapamycin as anti-cancer
Sehgal wasn’t ready to give up on rapamycin. On a hunch, he sent a sample of it to the National Cancer Institute (NCI), where it was screened for anti-cancer activity. Immunosuppressants are not usually good cancer treatments. In fact, the FDA requires most immunosuppressants to come with a warning stating that they increase the risk of cancer. The immune system is in charge of killing cancer cells. So, when you suppress the immune system, you increase the risk of cancer. Nevertheless, Seghal’s hunch paid off; the screening process revealed that rapamycin stopped the growth of cancer cell lines in a way that no other cancer treatment had ever done before.
Before the 1980s, chemotherapies had all been cytotoxic — that is, they killed living cells. This causes harsh side effects as healthy cells die as collateral damage. However, based on the NCI’s screening, rapamycin was cytostatic — in other words, it stopped cells from dividing but didn’t kill them. This discovery had the potential to radically change how cancer was treated. NCI quickly advanced rapamycin as a priority drug. Unfortunately, the drug was struck with more bad luck.
As Ayerst researchers and academic scientists planned clinical trials for rapamycin, Ayerst laid off 95% of its workforce. Despite its potential, the rapamycin program was aborted. However, once again, Sehgal wasn’t ready to give up. He took samples of rapamycin-producing bacterium home and placed them in his freezer, where they remained for six years.
Resurrection
In 1987, Ayerst merged with Wyeth, and new management took over. Sehgal convinced them to resurrect research into the drug and its anticancer effects. Following its revival, numerous reports confirmed rapamycin’s inhibitory effect on cell growth in everything from fungi to plants to animals. These inhibitory effects differed from organism to organism; however, the observation that rapamycin targets such a broad range of cells suggested that it acts through an evolutionarily important master regulator. This paved the way for pioneering studies in the 1990s that identified the highly conserved mechanistic target of rapamycin: mTOR, a protein important for cell division.
Following the discovery of mTOR, scientists found that the enzyme governs an intricate signaling network that controls virtually every aspect of growth and metabolism. mTOR decides if cells should multiply based on the level of amino acids, glucose, insulin, leptin, and oxygen. This assessment is critical. For example, if a cell begins multiplying without acquiring sufficient nutrients, it will die while trying to carry out the process.
With the discovery of mTOR, the FDA felt comfortable approving rapamycin for organ transplant patients in 1999, and it has been used by millions of patients since. Its immunosuppressive activity prevents the immune system from attacking transplanted organs, and its relative lack of side effects makes it much more tolerable for patients. Interestingly, researchers noticed that transplant patients who took rapamycin were less prone to developing cancer than other patients. Rapamycin was finally getting the attention it deserved. Unfortunately, rapamycin was hit with one final stroke of bad luck.
Surendra Sehgal, the scientist who never lost faith in rapamycin, was diagnosed with stage four colon cancer in 1998. At the time, colon cancer had a median survival time of 14.4 months. His physician began treating him with a rapamycin analog when the cancer spread to his liver, and the tumors in his liver never grew. He passed away in 2003, five years after his diagnosis. In 2004, an analog of rapamycin was approved to treat kidney cancer.
Rapamycin as anti-aging
As more research groups experimented with rapamycin and its analogs to suppress mTOR’s activity, they discovered that the drug extended the life spans of fungi and animals. As part of the National Institute on Aging’s Interventions Testing Program, researchers fed rapamycin to mice and studied how it altered their life spans. The results, reported in 2009, made headlines: Mice fed rapamycin lived an extra six months (equivalent to about 20 years in humans).
After the mouse study, scientists began exploring rapamycin’s anti-aging effects in other mammals, including people. In 2014, Novartis reported results from a trial in which 200 older adults took a pill containing either a placebo or one of three doses of a rapamycin analog (everolimus) for six weeks. The researchers didn’t want to wait decades to see if the treatment group lived longer, so they took a different approach to determine if rapamycin made elderly people more healthy.
mTOR is overactive as we age, which causes the immune system (and likely other systems) to work harder but not necessarily better. The researchers suspected that turning down mTOR activity would strengthen the study participants’ immune systems. After six weeks of treatment, the researchers gave all the study participants the flu vaccine and assessed how well the participants’ immune systems responded. Subjects on the lowest dose of everolimus had the most flu antibodies in their blood, suggesting the low dose improved their immune system’s function.
Immune system rejuvenation
It might seem contradictory that rapamycin, an immunosuppressant, can boost immune function. However, rapamycin was labeled an immunosuppressant at a period when scientists had only scratched the surface of how the immune system is regulated. At low doses, rapamycin “rejuvenates immunity,” calming hyper-immunity rather than suppressing healthy immunity. If rapamycin had been labeled an immunomodulator or anti-inflammatory drug, it would have sounded much more appealing.
Because rapamycin was lumped together with other immunosuppressants, it has an unfair reputation for increasing the risk of cancer and infections. However, studies have revealed that rapamycin prevents lymphoma and some types of cancer in transplant patients and improves resilience to pathogens. One crucial reason why the side effects of rapamycin are exaggerated is that the frequency of rapamycin side effects has often been estimated from studies lacking a healthy placebo group. Thankfully, this is beginning to change. In a placebo-controlled study in healthy elderly people, no side effects were noticed compared to placebo. In 2020, scientists at UCLA began a three-year study into rapamycin’s long-term safety and anti-aging effects in healthy older adults.
This article Rapamycin: The unlucky history of the most powerful anti-aging drug is featured on Big Think.
This content was originally published here.