Physicians at University of Utah Health revealed Wednesday they treated the third male in the U.S. to establish rare embolism that might have been triggered by the Johnson & & Johnson(J&J) COVID vaccine.
The guy, under age 50, is Utah’s very first believed case of vaccine-induced thrombotic thrombocytopenia (VITT), likewise called thrombosis and thrombocytopenia syndrome (TTS).
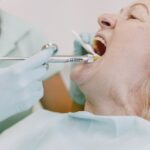
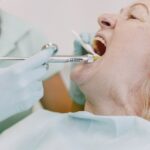
According to the medical center’s news release, the man received J&J’s vaccine in early April. Ten days later he experienced discomfort in his toes, which then progressed to his thighs. He later began experiencing chest discomfort.
According to KUTV2, medical professionals stated a CT scan exposed a bilateral pulmonary embolism. Physicians discovered low platelets and blood clots in his lungs and legs, leading them to suspect VITT was the cause.
Dr. Yazan Abou-Ismail, assistant teacher of medication in the medical center’s hematology department, dealt with the man. As Abou-Ismail carried out more tests, he began to strongly believe the embolism originated from the vaccine, though the Centers for Disease Controls and Prevention (CDC) is still investigating.
“There are certain cases where we would expect long-term results to take place,” Abou-Ismail stated. “I do not believe this is among those cases.”
The client was offered the advised treatment for VITT. Specialized medical tests followed the case and this diagnosis was reported to the Vaccine Adverse Event Reporting System and discussed directly with the CDC. The client responded to treatment and continues to recover in your home.
About-Ismail said the hospital is being transparent so the community can learn about VITT and acknowledge it earlier, but added he believes the threats of COVID outweigh prospective threats from the J&J vaccine.
VITT is identified by the presence of 2 conditions concurrently: (often in uncommon sites like the cerebral sinus veins or splanchnic veins) and, and has actually been connected to J&J and AstraZeneca COVID vaccines.
The J&J vaccine, marketed under the business’s Janssen subsidiary, and the AstraZeneca vaccine include replication-incompetent adenoviral vectors— human Ad26.COV2.S and chimpanzee ChAdOx1, respectively– which encode the spike glycoprotein on SARS-CoV-2.
It is thought that leak of DNA from the adenovirus-infected cells binds to platelet factor 4, triggering the production of auto-antibodies, according to Dr. Karen Furie, neurologist-in-chief at Rhode Island Hospital.
On April 23, the CDC’s independent advisory panel voted 10– 4 to advise the continued use of the J&J vaccine with no restrictions following a brief time out of the single-dose shot over blood clotting issues.
The CDC recognized 15 cases of blood clots, all in females, consisting of three deaths, seven hospitalizations and 5 recovering at home.
The CDC panel stated the link between blood clots and J&J’s vaccine was “possible,” however concluded the vaccine’s advantages surpassed the dangers, and recommended the vaccine for persons 18 years of age and older in the U.S. under the FDA’s Emergency Use Authorization.
Between Dec. 14, 2020 and April 23, VAERS consisted of a total of 1,845 reports of unfavorable occasions associated with blood clotting conditions. Of the 1,845 reports, 655 were credited to Pfizer, 577 to Moderna and 608 to J&J (593 cases more than the 15 acknowledged during the CDC’s April 23 meeting).
On April 27, The Defender reported a 30-year-old California man was hospitalized with embolism after getting J&J’s vaccine. It was the very first time U.S. public health officials specifically acknowledged VITT in a male who received J&J’s shot.
European regulators have identified links in between rare blood clots in both the AstraZeneca and J&J vaccine. On April 20, the European Medicines Agency stated a caution label should be added to J&J’s vaccine, but said general advantages still outweigh dangers.
On May 3, Denmark became the first European country to ditch the J&J vaccine, mentioning a possible link to embolism. The move by the Danish Health Authority followed its choice last month to discard AstraZeneca’s vaccine over comparable concerns.
The post Third U.S. Male Diagnosed With Vaccine-Induced Blood Clots After J&J Vaccine appeared first on Children’s Health Defense.
This material was initially published here.
, the male gotten J&J’s vaccine in early April. 10 days later he experienced discomfort in his toes, which then progressed to his thighs., assistant teacher of medicine in the medical center’s hematology division, treated the man. The patient was provided the advised treatment for VITT.

















![[New Webinar Starts Tomorrow] Discover Your Thyroid Type & 5 Completely False Things Doctors Say | Holistic Health Online](https://thehealthandwellnesscrier.com/wp-content/uploads/2023/02/New-Webinar-Starts-Tomorrow-Discover-Your-Thyroid-Type-5-Completely-False-Things-Doctors-Say-Holistic-Health-Online.png)