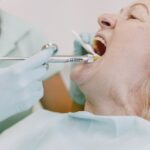
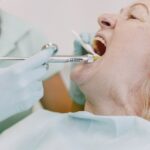
FDA Authorizes Pfizer Vaccine for 12-15-Year-Olds
The Pfizer vaccine has actually been authorized for kids ages 12 and up, expanding the U.S. population that will be safeguarded versus the virus and strengthening opportunities for a safe go back to full-time school in the fall, the Food and Drug Administration announced Monday.
“We know this is a big action for our nation. Vaccinating a younger population brings us closer to going back to a sense of normalcy and to ending the pandemic,” acting FDA Commissioner Janet Woodcock stated at a press briefing on Monday night.
Woodcock said guardians and moms and dads can “rest assured that in making our decision, the firm carried out a thorough and rigorous review of all available clinical data, as we have with all the COVID-19 vaccine permissions.”
Pfizer Vaccine Could Be Available For Ages 2 and Up as Early as This Fall, Exec Says
Data from Pfizer’s coronavirus vaccine trials in children aged two years and older could be offered as early as this fall, Pfizer’s Senior Vice President of Vaccine Clinical Research and Development Dr. William Gruber stated, perhaps paving the way for usage before the end of 2022.
The drugmaker is likewise performing scientific trials on their vaccine for children aged six months and older. Gruber prepares for data from those trials will likely be readily available “late at the end of the year or beginning of 2022.”
64 Days and Counting– Why Won’t the CDC Answer Our Questions?
On March 8, The Defender contacted the Centers for Disease Control and Prevention (CDC) with a written list of concerns about reported injuries and deaths associated with COVID vaccines. We inquired about how the CDC performs investigations into reported deaths, the status of ongoing examinations into injuries and deaths reported by the media, if autopsies were being conducted, the standard for figuring out whether an injury is causally connected to a vaccine, and education efforts to encourage and facilitate accurate and proper reporting …
It has been 64 days given that we sent our first email exploring VAERS data and reports, but still no action.
Clients Died From Neglect, Not COVID-19, in Ontario LTC Homes, Military Report Finds
Dozens of homeowners in 2 Ontario retirement home hit hard by the coronavirus passed away not from COVID-19 however from dehydration and overlook, the Canadian armed force says in reports obtained by The Globe and Mail.
The files contain new details about the deplorable conditions in 2 Toronto homes before the Forces stepped in last year, exposing for the first time that triggers besides COVID-19 accelerated the deaths of homeowners as break outs spiralled out of control and staffing collapsed.
At Downsview Long Term Care Centre, where one in four citizens caught the infection, another 26 died from dehydration before a military team got here last June to offer medical and humanitarian support.
Why FDA Should Not Authorize COVID Vaccines for Kids, Teens
For adults, the advantages of COVID-19 vaccination are enormous, while for kids, they are reasonably small. Unusual adverse effects from adult COVID-19 vaccination are unlikely to cause future vaccine hesitancy whose public health impact might be equivalent to the benefits of the adult COVID-19 vaccination program itself.
However accelerated mass kid vaccination under Emergency Use Authorization– possibly even spurred by school mandates and “vaccine passports“– provides a various balance of dangers and advantages. Uncommon adverse occasions actually could prove to be the most long lasting public health legacy of an EUA for kid COVID-19 vaccines.
Slovakia Suspends Use of Astrazeneca COVID-19 Vaccine as a Recipient Dies
Reuters via Yahoo! News reported:
Slovakia’s Health Ministry said on Tuesday it was suspending using AstraZeneca’s COVID-19 vaccine for individuals getting their very first dosages, after specialists evaluated the death of a recipient.
The state drug regulator, SUKL, said recently that the death of a 47-year-old woman was likely to be connected to the vaccine since of a predisposition that she had.
“The Health Ministry is thinking about various options at the minute, how to continue with this concern,” spokeswoman Zuzana Eliasova said. “At the moment, individuals waiting on their second dose are being inoculated with the AstraZeneca vaccine.”
A Misleading CDC Number
When the Centers for Disease Control and Prevention launched new guidelines last month for mask using, it announced that “less than 10 percent” of COVID-19 transmission was occurring outdoors. Media organizations duplicated the statistic, and it rapidly became a standard description of the frequency of outdoor transmission.
But the number is almost certainly misleading.
It seems based partially on a misclassification of some COVID transmission that really occurred in enclosed spaces (as I explain below). An even larger concern is the extreme caution of CDC authorities, who chose a standard– 10 percent– so high that nobody might fairly challenge it.
Brazil Agency Calls for Suspending AstraZeneca Vaccine for Pregnant Women
The Wall Street Journal reported:
Brazil’s health authority Anvisa has required the instant suspension of using AstraZeneca’s COVID-19 vaccine among pregnant women in the Latin American nation.
Anvisa stated late Monday that its recommendation was the outcome of “constant tracking of unfavorable events connected to Covid vaccines in usage in the country,” without providing further details.
The post appeared first on Children’s Health Defense.
This content was initially published here.
“We know this is a big step for our nation. The drugmaker is likewise performing medical trials on their vaccine for children aged six months and older.– provides a various balance of dangers and benefits.”The Health Ministry is considering numerous options at the minute, how to proceed with this issue,” spokesperson Zuzana Eliasova said. It appears to be based partly on a misclassification of some COVID transmission that actually took location in enclosed areas (as I discuss listed below).