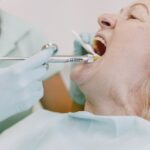
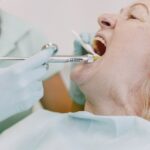
This Blood Test Is Over 90 Percent Accurate in Diagnosing Alzheimer’s Disease The new diagnostic is fast and noninvasive compared with existing options, study finds. A novel blood test may offer a relatively easy way to identify Alzheimer’s disease in its early stages, when treatments can be most effective. Findings published this month in JAMA Neurology indicate that the blood exam, which measures a specific protein in the plasma, has an accuracy rate of up to 95 percent in detecting the presence of this most common type of dementia. The test identifies phosphorylated TAU 217 protein (p-tau217), which increases in the body at the same time as beta-amyloid and tau proteins accumulate in the brain. A buildup of beta-amyloid has long been recognized as a hallmark of Alzheimer’s disease, according to the Alzheimer’s Association. Study authors found the precision of the p-tau217 test to be comparable to the current “gold standard” methods for diagnosing Alzheimer’s: positron emission tomography (PET) imaging and lumbar puncture (or spinal tap, as detailed by InformedHealth.org), which is used to sample and analyze cerebrospinal fluid. “PET scans, however, are not widely available in all communities, and they require an injection of a radioactive material to see the amyloid and tau in the brain, while spinal taps are invasive and time-consuming,”says Howard Fillit, MD, chief science officer with the Alzheimer’s Drug Discovery Foundation. “The value of the blood test is that it can be performed in the course of routine clinical care by doctors who do blood tests all the time in their office.” Blood tests are also less expensive and faster to administer. A Definitive Diagnosis for 8 out of 10 Tested For the study, blood samples, brain scans and spinal taps were taken from 786 adults in the United States, Canada, and Spain. The average participant was 66 years old, and one-third were classified as cognitively impaired. When scientists compared the p-tau217 blood test to the spinal taps and PET scans, they found that the results indicating brain changes associated with Alzheimer’s disease were on a par with those determined by spinal fluid and PET scan. Researchers noted that approximately 20 percent of the study subjects had p-tau217 blood test results that were uncertain, and they would require spinal taps or PET scans to diagnose whether they had Alzheimer’s. About 80 percent, however, had definitive results, which would decrease the number of tests needed to confirm the diagnosis. The results also indicated that a greater increase in p-tau217 corresponded to a higher degree of cognitive impairment. Still, Andreas Jeromin, PhD, a study author and chief scientific officer at ALZpath, the company which developed the test, called ALZpath Dx, underscored that this tool is intended to be used as part of an overall patient evaluation. “It’s not really standalone,” he says. “The results will be explained to the patient along with the overall assessment of clinical presentation [of the disease].” Why Early Detection of Alzheimer’s Is So Important Dr. Jeromin suggests that initially the test will most likely be used in patients showing some signs of mild cognitive impairment and early Alzheimer’s disease, such as forgetfulness and asking questions repeatedly, per the NHS. “The earlier you treat the disease with pharmacological interventions and modifications of lifestyle, the higher the likelihood that they will slow down the progression of cognitive decline,” says Jeromin. “That’s why it’s important to assess these people as early as possible.” Early diagnosis could make treatment with new drugs, such as lecanemab (Leqembi), more effective. The U.S. Food and Drug Administration approved the monoclonal antibody medication last year after study results showed that it reduced the amount of amyloid in the brain and slowed cognitive decline. Lifestyle modifications are more difficult to measure, but diet, exercise, socializing, and activities that promote cognitive ability may all help slow decline, according to Jeromin. He suggests that learning a language or reading books are types of activities that may improve a person’s disease trajectory. Although the test is currently for use only in research studies, ALZpath Dx will be made available as laboratory-developed test (LDT) in the first partner lab in the upcoming weeks, with additional labs in the United States to follow throughout 2024. Patients will be able to order the test from anywhere in the US via a physician, at a cost estimated to be between $200 and $500. Jeromin anticipates that FDA approval of the test as a diagnostics tool may come in late 2025 or early 2026. In the future, he envisions that the test could be used as a part of any standard wellness visit, just as we test for diabetes and other chronic diseases. The test may also be used to measure how well Alzheimer’s medications and other interventions are working in patients. A Promising Time for Alzheimer’s Treatment and Evaluation Alzheimer’s is an irreversible, progressive brain disorder affecting more than 6.5 million Americans that slowly destroys memory and thinking skills and, eventually, the ability to carry out simple tasks. But now is an encouraging time for diagnosis and treatment of Alzheimer’s disease, according to Rebecca Edelmayer, PhD, the senior director of scientific engagement for the Alzheimer’s Association. ALZpath Dx is just one of several Alzheimer’s blood tests that are under development for clinical use. “We are quickly moving toward a time when we’ll have validated diagnostic tools that allow for earlier and more accurate diagnosis in broader clinical settings,” she says. “However, there remain unanswered questions around the generalizability and broad use of blood tests for Alzheimer’s in primary care.” The Alzheimer’s Association stresses that when considering any testing for Alzheimer’s or dementia, it’s important to have a conversation with your healthcare provider to see if you are a candidate. The Alzheimer’s Association provides official recommendations on how blood tests and blood biomarkers should be used. “The results we’re seeing with this blood biomarker test are encouraging,” says Dr. Edelmayer. “[But] at this time, available blood tests are best used as an initial test to aid in diagnosis, and may lead to further cognitive testing and additional biomarker testing using CSF [cerobrospinal fluid from a spinal tap] or PET scans to confirm a diagnosis.” Editorial Sources and Fact-Checking Everyday Health follows strict sourcing guidelines to ensure the accuracy of its content, outlined in our editorial policy. We use only trustworthy sources, including peer-reviewed studies, board-certified medical experts, patients with lived experience, and information from top institutions. Resources Ashton N et al. Diagnostic Accuracy of a Plasma Phosphorylated Tau 217 Immunoassay for Alzheimer Disease Pathology. JAMA Neurology . January 22, 2024. Beta-amyloid and Alzheimer’s Disease. Alzheimer’s Association. May 2023. Hansson O et al. The Alzheimer’s Association Appropriate Use Recommendations for Blood Biomarkers in Alzheimer’s Disease. Alzheimer’s and Dementia . July 31, 2022. What Happens During a Lumbar Puncture (Spinal Tap)? InformedHealth.org. May 23, 2016. Alzheimer’s Disease — Symtoms. NHS. July 5, 2021.
This content was originally published here.